IWILFIN Study Results
How IWILFIN was studied
IWILFIN’s effectiveness was assessed in Study 3b, which included pediatric high-risk neuroblastoma patients in remission following completion of upfront therapy. Patients in remission received IWILFIN twice daily for up to 2 years.
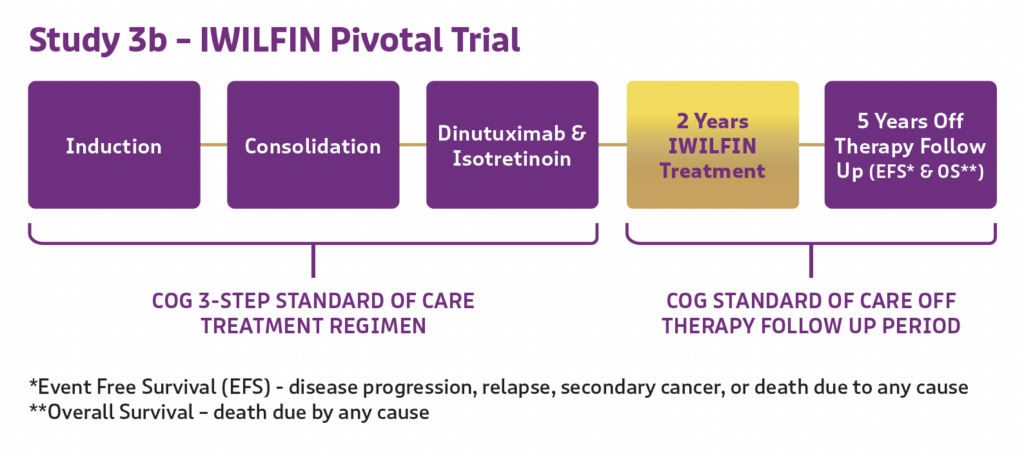
Results were compared with an external control database from Study ANBL0032, where patients received the same standard of care regimen up until they received dinutuximab instead of IWILFIN.
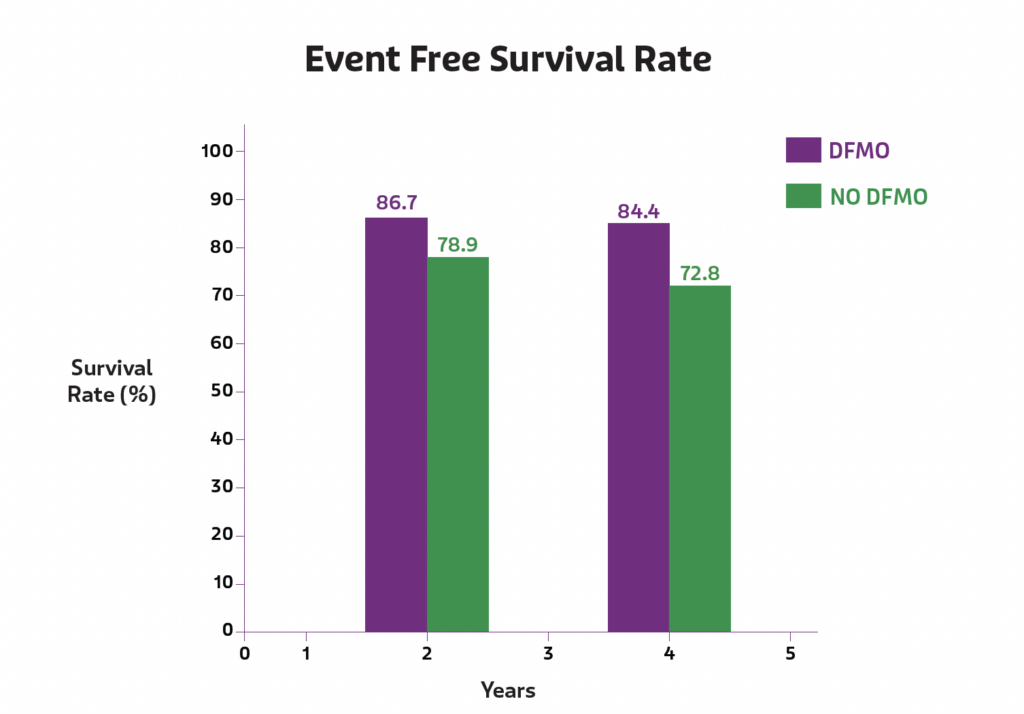
Improvement in event free survival
In studies, children taking IWILFIN achieved a 52% reduction in the risk of high-risk neuroblastoma relapse
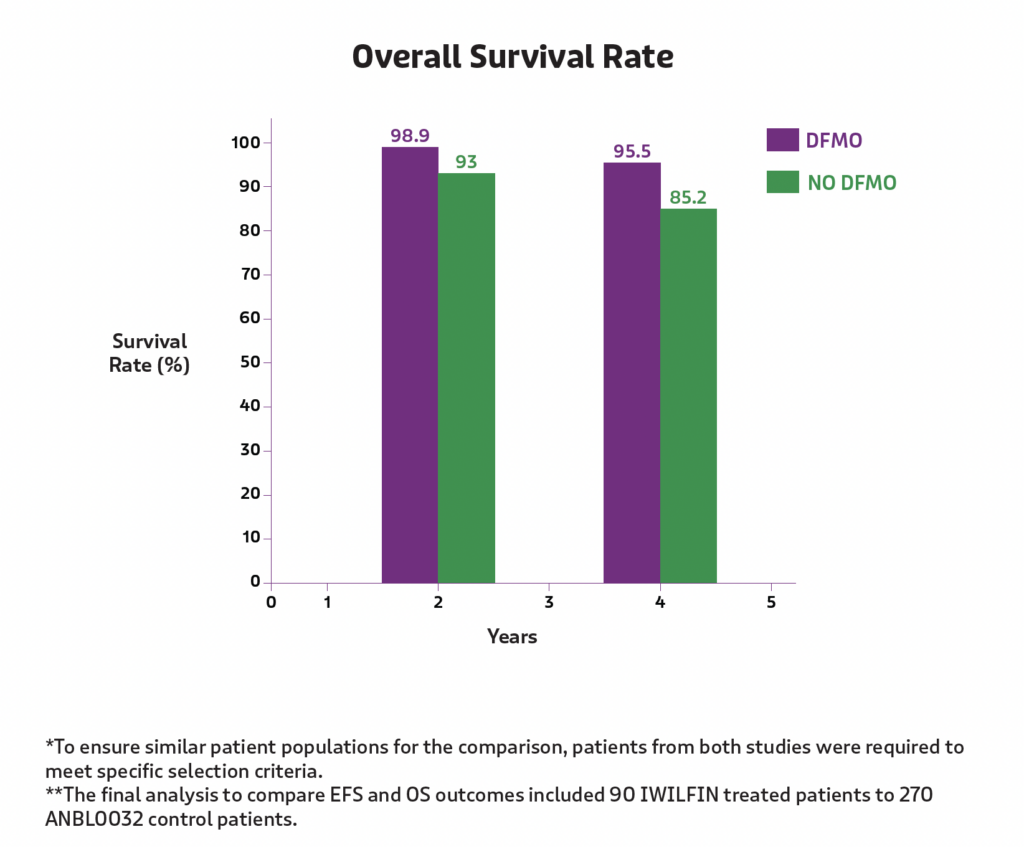
Improvement in overall survival
Overall, 92% of the children treated with IWILFIN were still alive after 5-years post remission, compared to 79% of the children in the external control group